Gases
Learn about the properties and behavior of gases in physics.
Understanding Gases
Gas is the state where particles are widely spaced, move rapidly, and have no fixed shape or volume. This means that they will fill a entire container they are placed in.
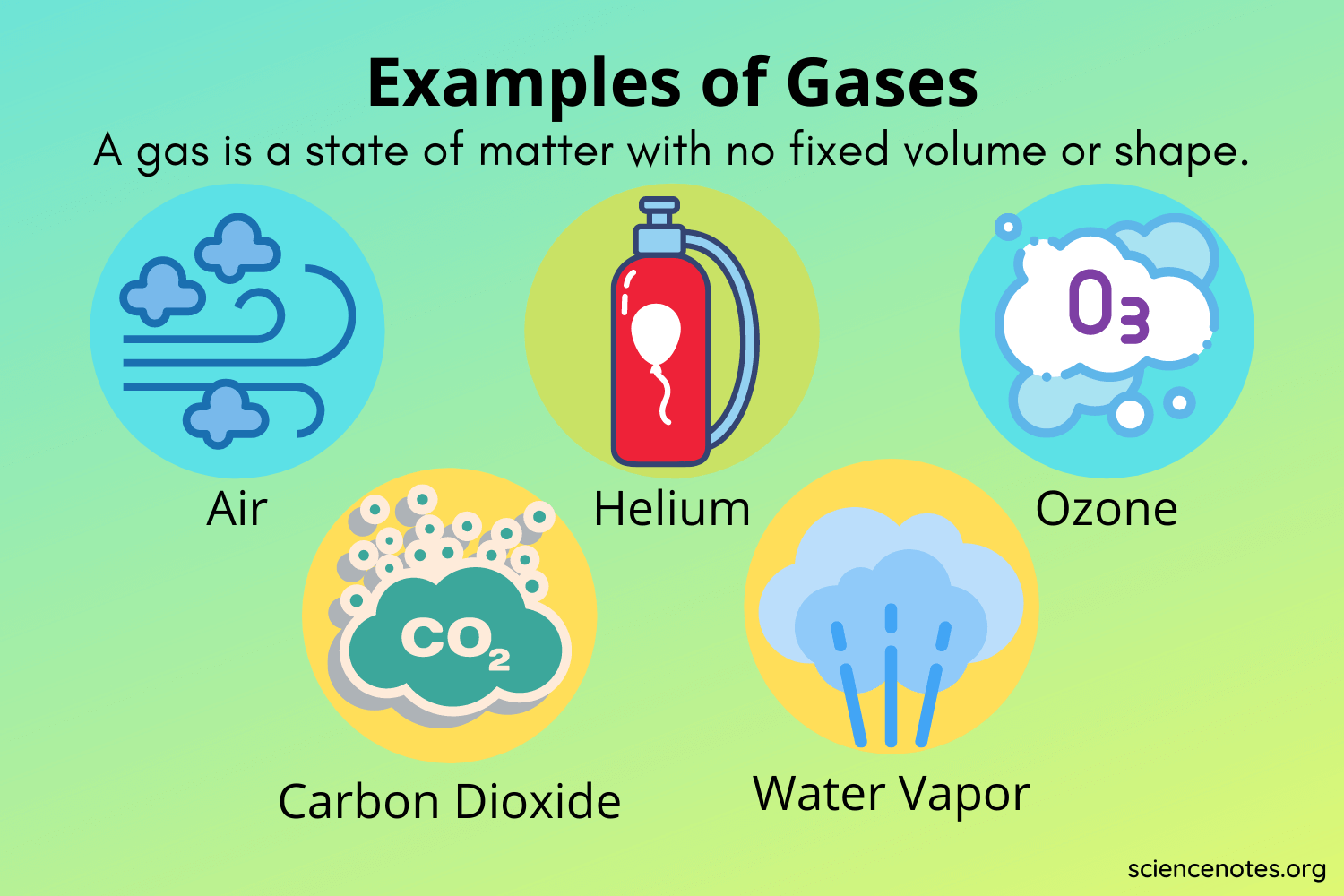
Common examples include:
- Oxygen
- Carbon Dioxide
- Helium
- Nitrogen
- Steam
Key Properties of Gases
Volume & Shape
Gases expand to fill any container, taking both its shape and volume. This is due to the high kinetic energy of gas particles.
Compressibility
Highly compressible due to large spaces between particles, making them ideal for storage and transport.
Diffusion
Gas particles spread out quickly and mix with other gases, explained by their rapid, random motion.
Pressure
Exert pressure equally in all directions due to constant particle collisions with container walls.
Gas Behavior
Temperature Effects
- Increased temperature = higher particle speed
- Volume expands with heating
- Pressure increases with temperature (fixed volume)
Gas Laws
- Boyle's Law: Pressure-Volume relationship
- Charles's Law: Volume-Temperature relationship
- Ideal Gas Law: PV = nRT
Real-World Applications
Industrial Uses
Natural gas for energy, nitrogen for food preservation, helium for cooling.
Medical Applications
Oxygen for breathing support, anesthetic gases, sterilization processes.
Environmental Impact
Atmospheric composition, greenhouse gases, air quality monitoring.
